

Suitable for fixation of ligaments or tendons to bone in orthopedic reconstruction surgery.
Item No :
GOHE028MOQ :
10 PiecesClassification :
Class IIIColor :
No Color / Custom ColorOrigin :
Xiamen, ChinaPayment :
T/T 50% and balance before shipmentLead Time :
Depends on the order circumstancesLooped Titanium Plate Fixation System For Orthopedic Surgery
The looped titanium plate fixation system is divided into adjustable and non-adjustable types according to its structural form. The product consists of a titanium plate, a coil, and sutures. The titanium plate is made of titanium alloy TC4 material, with a surface that is either uncolored or subjected to colored anodizing treatment. The coil and sutures are made of ultra-high molecular weight polyethylene. The coil is available in both adjustable and non-adjustable types and is white in color. The sutures are divided into traction sutures and loop sutures, which are blue and blue-white in color, respectively. These sutures are not implanted into the human body. The dye used for the sutures is cobalt-chrome blue, and the surface of the sutures is free of coatings. The product is sterilized with ethylene oxide.
Ligament Fixation Titanium Plate Features:
- Enhanced Durability: The rounded edges of the threading holes reduce wear on sutures, enhancing the product's durability;
- Versatile Options: The adjustable design shortens surgery time and boosts efficiency compared to the fixed type;
- Biocompatibility: Made of titanium, it is highly biocompatible. Its small size ensures strong fixation while preventing soft tissue inflammation;
- Reduced Inventory Needs: The adjustable coil length means fewer specifications are needed in stock, simplifying inventory management.
ACL/PCL Fixation Device Images:
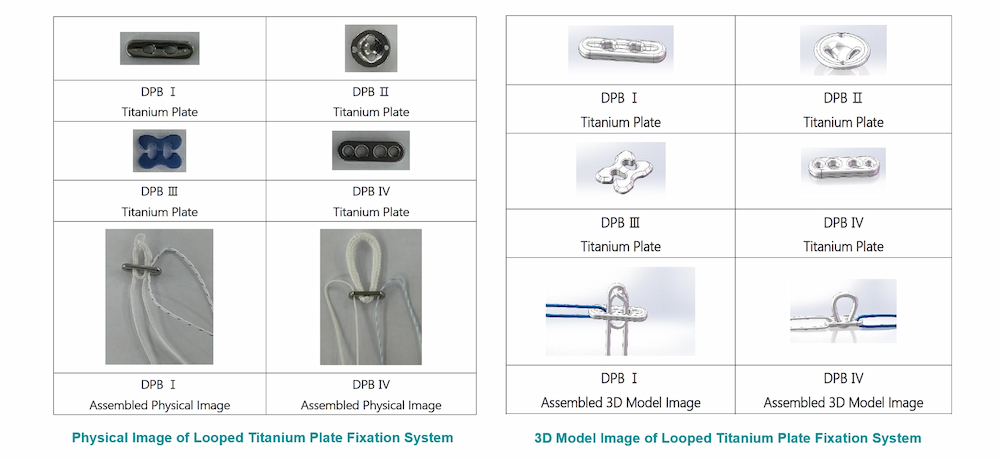
Looped Titanium Plate Specifications:
| Model Number | Product Description |
| DPB Ⅰ | Titanium plate, adjustable loop (white), one No. 5 traction suture (blue-white) |
| DPB Ⅱ | 14mm |
| DPB Ⅲ | 6mm、8mm |
| DPB Ⅳ 20 | Titanium plate, 20mm non-adjustable loop (white), one No. 5 traction suture (blue-white), and one No. 2 flipping suture (blue) |
| DPB Ⅳ 25 | Titanium plate, 25mm non-adjustable loop (white), one No. 5 traction suture (blue-white), and one No. 2 flipping suture (blue) |
| DPB Ⅳ 30 | Titanium plate, 30mm non-adjustable loop (white), one No. 5 traction suture (blue-white), and one No. 2 flipping suture (blue) |
| DPB Ⅳ 35 | Titanium plate, 35mm non-adjustable loop (white), one No. 5 traction suture (blue-white), and one No. 2 flipping suture (blue) |
| DPB Ⅳ 40 | Titanium plate, 40mm non-adjustable loop (white), one No. 5 traction suture (blue-white), and one No. 2 flipping suture (blue) |
| DPB Ⅳ 45 | Titanium plate, 45mm non-adjustable loop (white), one No. 5 traction suture (blue-white), and one No. 2 flipping suture (blue) |
| DPB Ⅳ 50 | Titanium plate, 50mm non-adjustable loop (white), one No. 5 traction suture (blue-white), and one No. 2 flipping suture (blue) |
| DPB Ⅳ 55 | Titanium plate, 55mm non-adjustable loop (white), one No. 5 traction suture (blue-white), and one No. 2 flipping suture (blue) |
| DPB Ⅳ 60 | Titanium plate, 60mm non-adjustable loop (white), one No. 5 traction suture (blue-white), and one No. 2 flipping suture (blue) |
1. The product is intended for use by orthopedic specialists only. The implantation process and conditions of the product should comply with the relevant technical management standards. The applicability requirements must be fully considered during clinical use;
2. The physician should assess the patient preoperatively and determine whether the patient is suitable for the use of this product based on the patient's condition. The appropriate model and size of the product should be selected;
3. Before use, the product should be verified. It is essential to check the labels and indicated specifications on the packaging to avoid misuse;
4. This product is a single-use implant and must not be reused;
5. This product is provided in a sterile manner. Do not resterilize;
6. Do not use expired or products with damaged primary packaging;
7. The product is not intended for ligament replacement;
8. Do not trim the sutures to a length shorter than 6 mm;
9. The product has not been tested or evaluated for temperature rise, displacement, or artifact generation in a magnetic resonance imaging (MRI) environment. Consult your physician before undergoing CT, MRI, or other examinations.